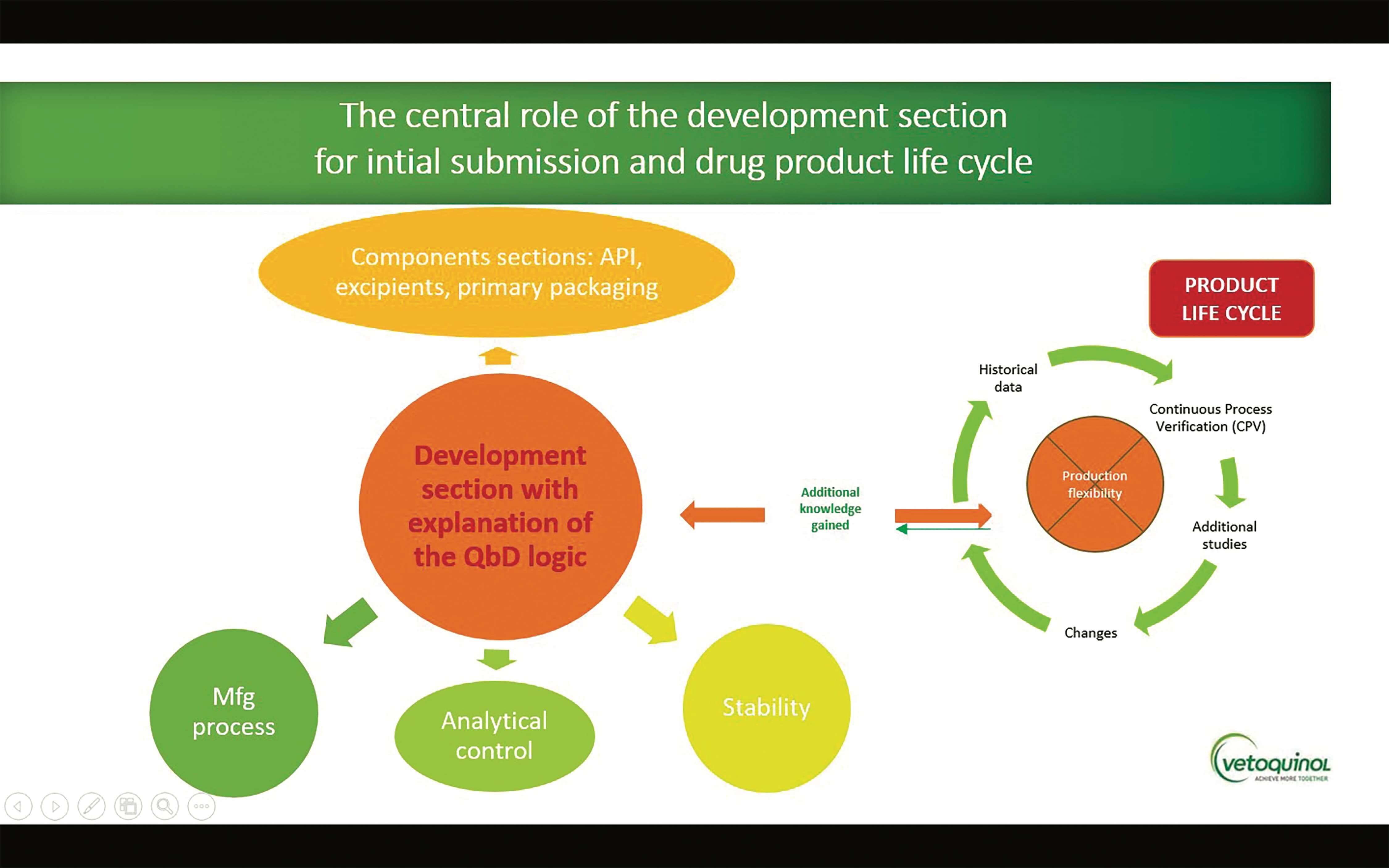
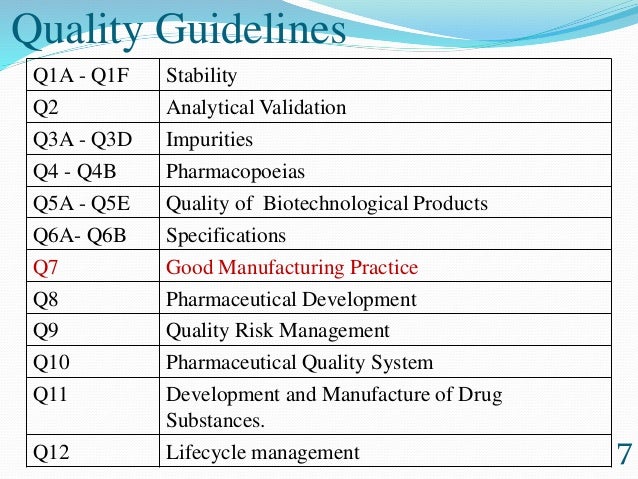
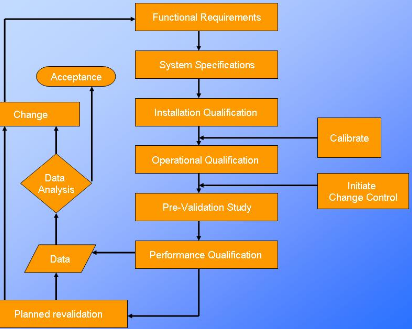
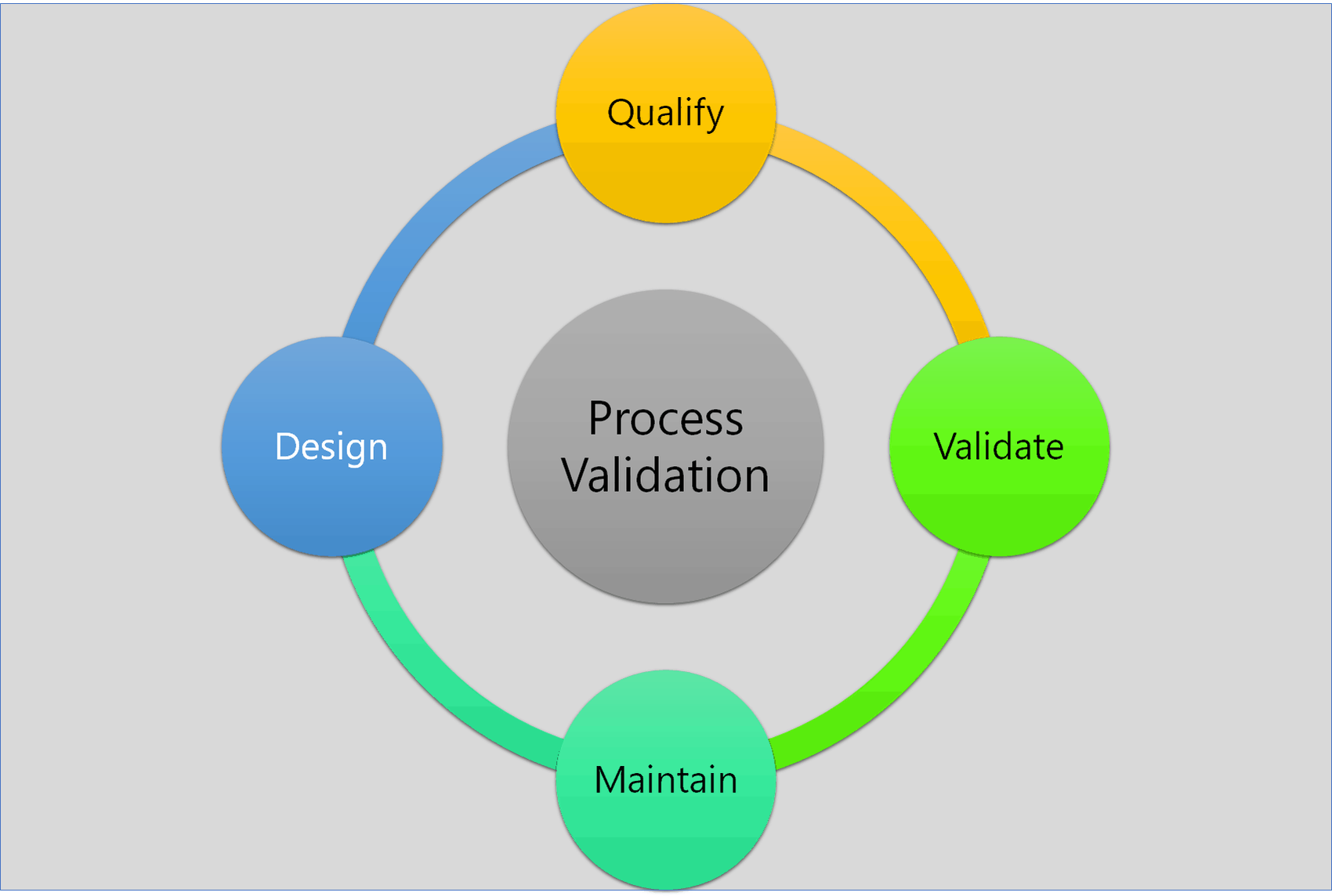
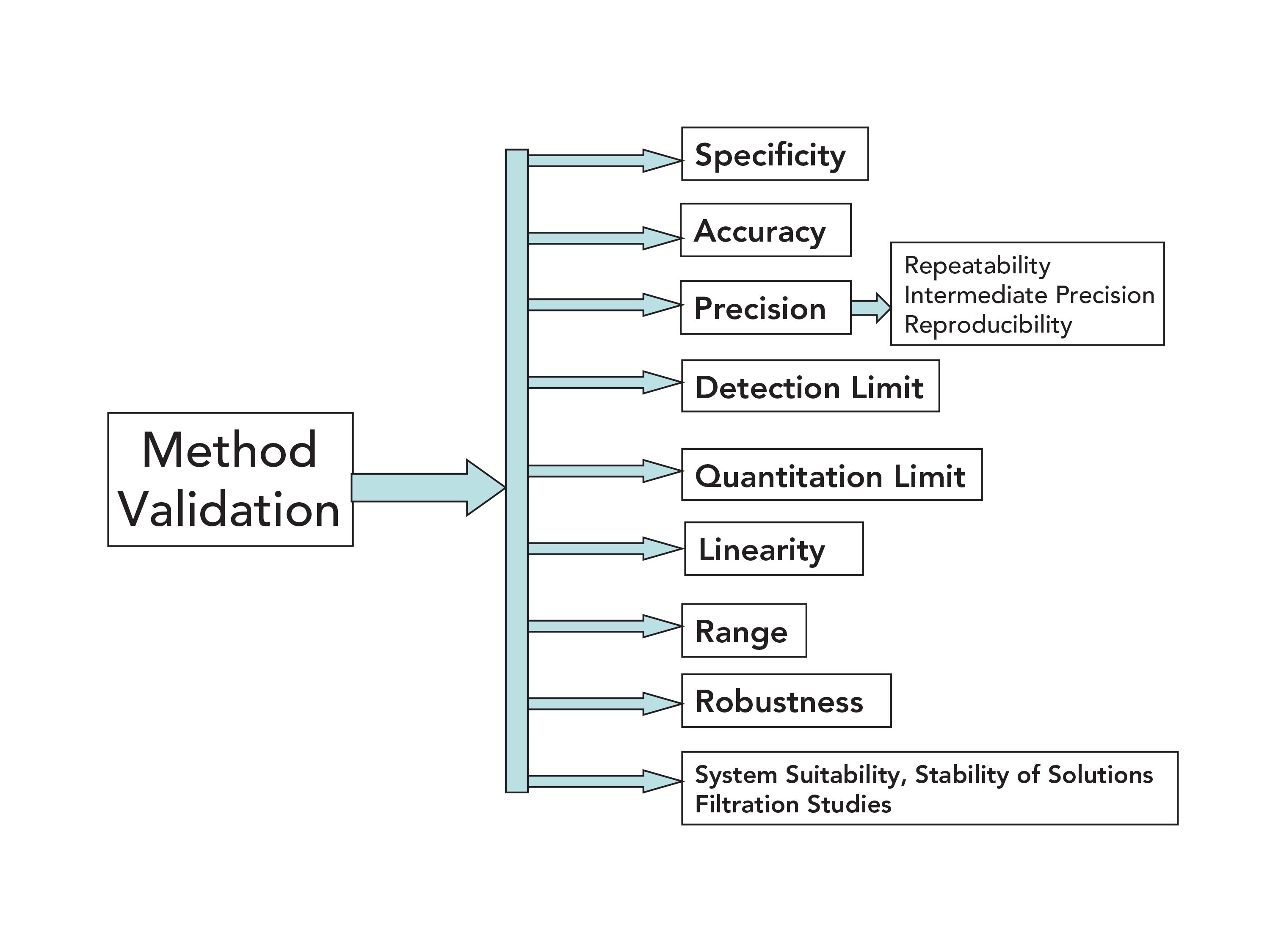
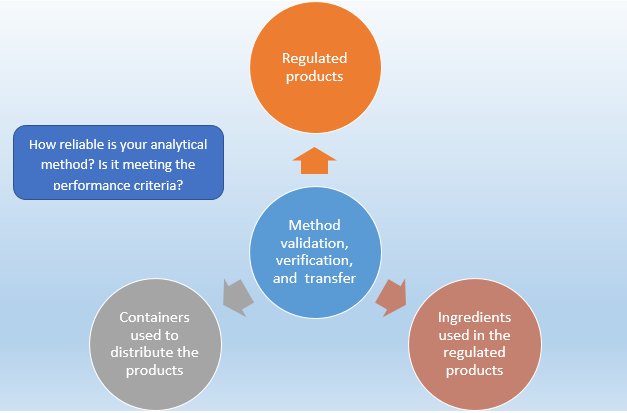
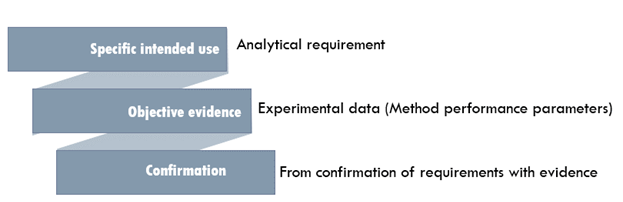
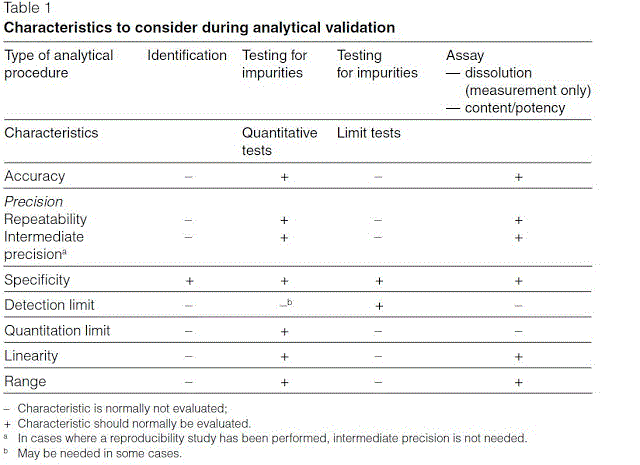
Analytical Method Validation for Quality Assurance and Process Validation Professionals | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology

Integrated Method Development and Validation Dr. Ludwig Huber RACI Conference - Chemical Analyses. - ppt download

Analytical Method Validation for Quality Assurance and Process Validation Professionals | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology